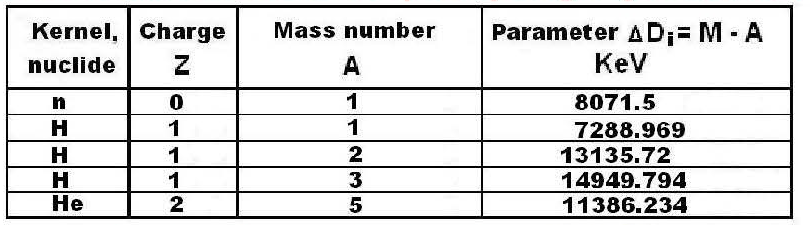
Since the parameter ΔDi in the table № A-1 is given in terms of energy its formula looks like:
ΔDi = Mi . C2– Ai . C2 (3.12)
From the formula (3.12) we define value of weight in terms of energy Mi . C2:
Mi . C2= ΔDi + Ai . C2 (3.13)
Hence: ΔDa = Ma . C2– Aa . C2;
ΔDb = Mb . C2– Ab . C2 ;
ΔDc = Mc . C2– Ac . C2 ;
Ma . C2= ΔDa + Aa . C2 ;
Mb . C2= ΔDb + Ab . C2 ;
Mc . C2= ΔDc + Ac . C2 (3.14)
Let’s substitute formulas (3.14) in (3.3) and (3.4) or in (3.8):
E2с = ΔМ2с . C2= Ma . C2+ Mb . C2— Mc . C2
E2с = (ΔDa + Aa . C2) + (ΔDb + Ab . C2) — (ΔDc + Ac . C2)
As a result of condition (3.11), after reduction we shall receive
E2с = ΔDa + ΔDb —ΔDc , (3.15)
That corresponds with the formula (3.10).
After similar transformations with parameter Esv we shall receive the formula
-E2с= Esva+ Esvb — Esvc (3.16)
That corresponds to the formula (3.9).
For comparison, in the table №T-3.3, in lines 15, 15a, 15b; 16, 16a, 16b the results of calculations of allocated energy E2с are resulted at the moment synthesis of kernels of carbon 176C and nitrogen 177N, by three ways E2с=ΔDa+ΔDb –ΔDc — in lines 15,16;
E2с = ΔМ2с . C2= Ma . C2+ Mb . C2— Mc . C2— in lines 15a, 16a;
And -E2с= Esva+ Esvb — Esvc— in lines 15b, 16b.
From a comparison of the received data in lines 15, 15a, 15b; 16, 16a, 16b it is visible, that divergences in the results are significant, and in further calculations we shall use formulas (3.10) and (3.15).
(3.10)
At j=2 E2с=ΔDa+ΔDb —ΔDc (3.15)
It is possible to define the energy allocated in the time of synthesis of kernels or nuclides under the formulas:
(3.17)
In table № A-1 the data ΔD is presented considering the weight of electronic environments of atoms, but at calculation of formulas (3.10) and (3.15) the weights of all electrons are completely reduced.
— Assumptions
We have defined formulas on which we shall count the allocated energy in time of synthesis of kernels. But for the continuation of the analysis it is necessary for us to make a number of assumptions since today it’s impossible to establish precisely which chains present in the synthesis in stars, how many kernels are participated in one act of synthesis, and under what conditions the quantity of kernels participating in one act varies.
Considering the wide scatter of stars weight, and the huge quantity of kernels participating in the synthesis in each star, it is possible to make a number of confident assumptions:
— Any variants, of chains of synthesis of kernels, without restriction are possible;
— Any quantity of kernels or nuclides participating in one act of synthesis from two j=2 and up to j=Ac (Ai) is possible;
— It is possible that the synthesis of kernels with the mass numbers which are located at the end of the periodic table of elements and above Ai > 250, but unfortunately, we are limited by the knowledge in the limits of this table, therefore chains of the synthesis of kernels will be considered only by it.
For simplification of the maintenance of the analysis we accept, that during the time of synthesis, the sum of protons and neutrons of kernels a and b participated in the synthesis is equal to the number of protons and the neutrons of the received kernel c (a condition (3.11)).
— A choice of a way of the analysis
On an example of the synthesis of two kernels or nuclides we shall consider the chosen way of the analysis.
In the beginning it is necessary for us to choose a chain of synthesis.
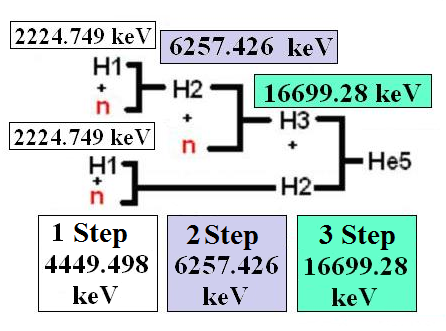
Let’s take for an example a chain from hydrogen with Z=1 and A=1 up to helium with Z=2 and A=5, as shown in the scheme № S-3.1.
Chain of synthesis of a kernel of helium with Z=2 and A=5 from two kernels or nuclides
Participating in one act of synthesis.
(13) Scheme № S-3.1.
Let’s make the table of nuclides and kernels participated in the synthesis of a kernel of helium He-5.
(14) Table № T-3.1.
Parameters of nuclides and the kernels participating in synthesis of a kernel of helium He-5.
Let’s make formulas of synthesis under the scheme № S-3.1:
1. 11H+10n=21H+E2H2
Where E2H2— the energy allocated in the time of synthesis 11H and 10n.
E2H2 = ΔDH1+ΔDn- ΔDH2=7288.969+8071.5-13135.72=2224.749 KeV.
2. 21H+10n=31H+E2H3
Where E2H3 — the energy allocated in the time of synthesis 21H and 10n.
E2H3 = ΔDH2+ΔDn- ΔDH3=13135.72+8071.5-14949.794=6257.426 KeV.
3. 31H+21H=52He +E2He5
Where E2He5 — the energy allocated in the time of synthesis31H and21H.
E2He5= ΔDH3+ΔD H2 — ΔDHe5=14949.794+13135.72-11386.234=16699.28 KeV.
Except for the value of energy allocated in one act of synthesis we interested in the value of the allocated energy in a step. The accessory of the reaction to one step or another is defined by the quantity of reactions of the synthesis which has passed the given kernel from a state when there were free protons and the neutrons included in its structure.
From the scheme № S-3.1 it is visible, that the synthesis of kernel He-5 goes three steps.
At the first step two reactions of synthesis occurs
11H+10n=21H+2224.749 KeV
11H+10n=21H+2224.749 KeV